Q&A
1. What is a generic medicine?
A generic medicine contains the same active ingredient as the originator product on which it is based and as such is interchangeable with this originator. It offers the same high quality and efficacy, together with affordability. This combination has made them increasingly attractive for healthcare systems as a whole and patients in particular. They can be marketed after the patent expiry of the originator product on which they are based.
2. Why are generic medicines regarded as equivalent to originator medicines?
Generic medicines contain the same active ingredient – the sole driving force behind a medicine’s therapeutic action – as the originators on which they are based. Whilst they might contain additional, non-active ingredients and differ in size, colour or shape, they are subject to the same rigorous authorisation process as the originator products on which they are based.
Furthermore, a generic product can only qualify for authorisation if it is “bioequivalent” to the originator product—i.e. it must work in essentially the same way in the patient’s body.
3. What is bioequivalence?
The key factor in creating a generic medicine is establishing bioequivalence. Bioequivalence means that, when compared scientifically, the generic medicine and the originator product demonstrate essentially the same rate and extent of biological availability of the active substance in the body when administered in the same dose. In simple terms, the generic medicine and the original product must be equally effective.
4. How are the quality, safety and efficacy of medicines ensured in the EU?
In the EU all medicines are subject to rigorous assessments by medicines authorities before they can be authorised for use in patients. The tests for generic medicines – the same that are applied to originator products – must confirm a high level of safety, quality and efficacy. They can either be carried out at national level, in the Member States, or through the Amsterdam based European Medicines Agency (EMA).
5. Are there other safety mechanisms in place for generic medicines?
Yes. Because they are treated in the same way as originator products, manufacturing plants for generic medicines are also subject to Good Manufacturing Practice (GMP) inspections. Generics manufacturers are also obliged to monitor their products continuously once they have been placed on the market, in order to report any adverse reactions to regulatory authorities.
6. How do generic medicines benefit both patients and healthcare budgets?
In an era when increasing demands are being made on Europe’s healthcare services, generic medicines provide a major benefit to society by ensuring patient access to quality, safe and effective medicines while reducing the cost of pharmaceutical care. This can often save purchasers between 20-90% of the total cost, whilst ensuring therapeutic equivalence.
Payers within healthcare systems know that they can offer quality care, whilst realising savings, which can then be channelled back into the healthcare sector to help finance more expensive therapies. Moreover, competition from generics also encourages originator manufacturers not only to lower their own prices, but also to invest in new research.
The increasing life expectancy of the European population significantly stresses the importance of generic medicines potential. Without the savings that generic medicines treatments offer, Europe would not be in a position to ensure access to quality, safe and affordable treatment to all its citizens.
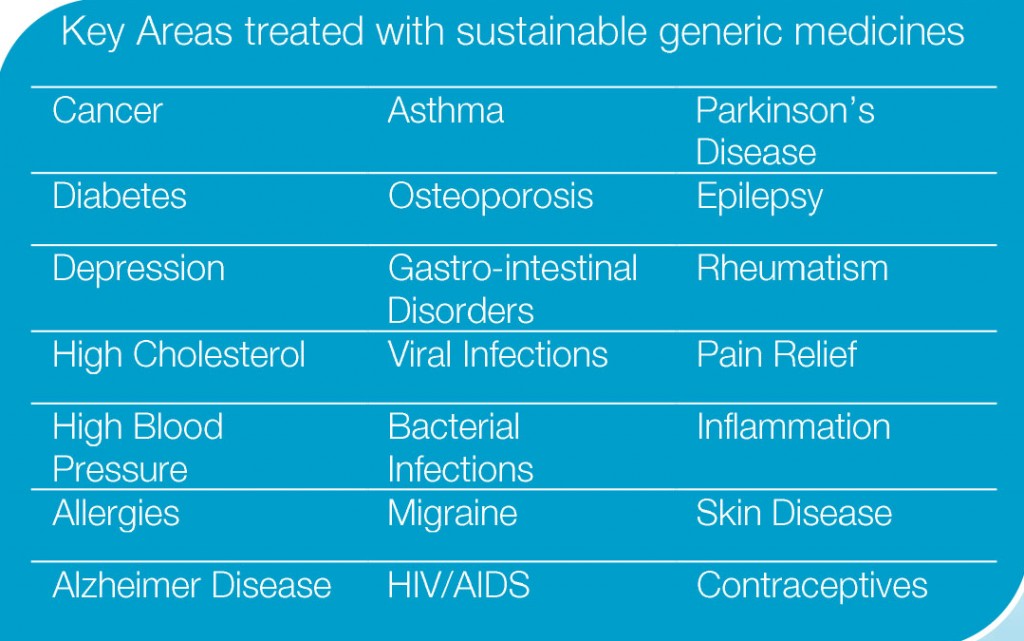
7. What are the key treatment areas treated by generic medicines?
Where a generic medicine is based on an originator product, it can treat exactly the same condition with the same high level of quality, safety and efficacy, often at a much lower cost. Generic medicines are therefore guaranteeing the highest level of quality care while able to offer savings across the entire healthcare spectrum.
8. How long does patent protection last?
Standard patent protection in the EU lasts for 20 years, although the pharmaceutical sector is unique in that this protection can be extended by a Supplementary Protection Certificate (SPC). This is designed to compensate manufacturers for the period between the filing of a patent application for a new medicinal product and authorisation to place it on the market. Generic medicines can be made available to patients in the EU only after the relevant patents and SPCs on the originator product have expired.
9. Can a medicinal product have more than one patent?
Yes. Pharmaceutical products are often covered by a number of patents, sometimes up to 40 or more. In addition, a patent on a new use (“indication”) formulation, salt or ester can block the registration or marketing of a generic medicine for treatments where the basic patent has already expired. This is a strategy known as “evergreening” which aims to prevent or delay competition from generic medicines by extending market protection through patents on minor changes to the original product. Lower patent standards also allow companies to set up “patent thickets” to safeguard their products from competition.
10. What is the additional protection of “data exclusivity”?
Introduced in 1987, data exclusivity exists separately from patent protection for originator medicines and was designed to compensate manufacturers for insufficient product patent protection in some countries. For the period of its duration, generics manufacturers may not apply for a market authorisation. Ironically, although patent protection is now strong in EU Member States, data exclusivity was maintained in the EU pharmaceutical legislation implemented in 2005.
11. How quickly can patients access generic medicines after protection expires?
The registration of a generic medicine usually takes 1 to 2 years, but there can be many reasons for extended delays to market. For example, similarly to originator products, generics are subject to pricing and reimbursement procedures. Moreover, far from being simple “copies” of originators, many generics undergo additional development, which may postpone marketing beyond the initial commercial decision to do so.
12. Can generic medicines manufacturers use data from originator products?
No, generic medicines applications do not make use of any data from the originator registration file, because this is never revealed to third parties. Instead, generic medicines producers must research and develop their own formulation of the product, which must then be approved under the same EU requirements as originals, with the additional confirmation of bioequivalence. Since generic medicinal products contain good quality substances, the pre-clinical tests and clinical trials performed by the originator are not repeated, as this would be both unethical and contrary to international convention. The safety and efficacy of a generic product is cross referenced with the originator product’s dossier by the medicines authorities, who alone have access to these files.



