



The European medicines industry has extensive manufacturing capabilities in producing high quality generic, biosimilar and value added medicines throughout Europe. With over 400 manufacturing plants and 126 R&D centres in Europe, companies represented within Medicines for Europe provide over 190 000 skilled, high value direct jobs in Europe, and more than half a million indirect jobs. Medicines for Europe is committed to supporting industrial policy initiatives aimed at strengthening the European industrial base, increasing investments in R&D for generic, biosimilar and value added medicines, boosting EU competitiveness, and taking up the opportunities which are opening up in Europe and worldwide.
Europe must prioritise public health and access to medicines recognising the value of of a resilient and strong European generic, biosimilar and value added medicines industry and the need to increase investments in this field. We believe that manufacturing leadership and supply chain resilience rely on smart policymaking based on three key factors:
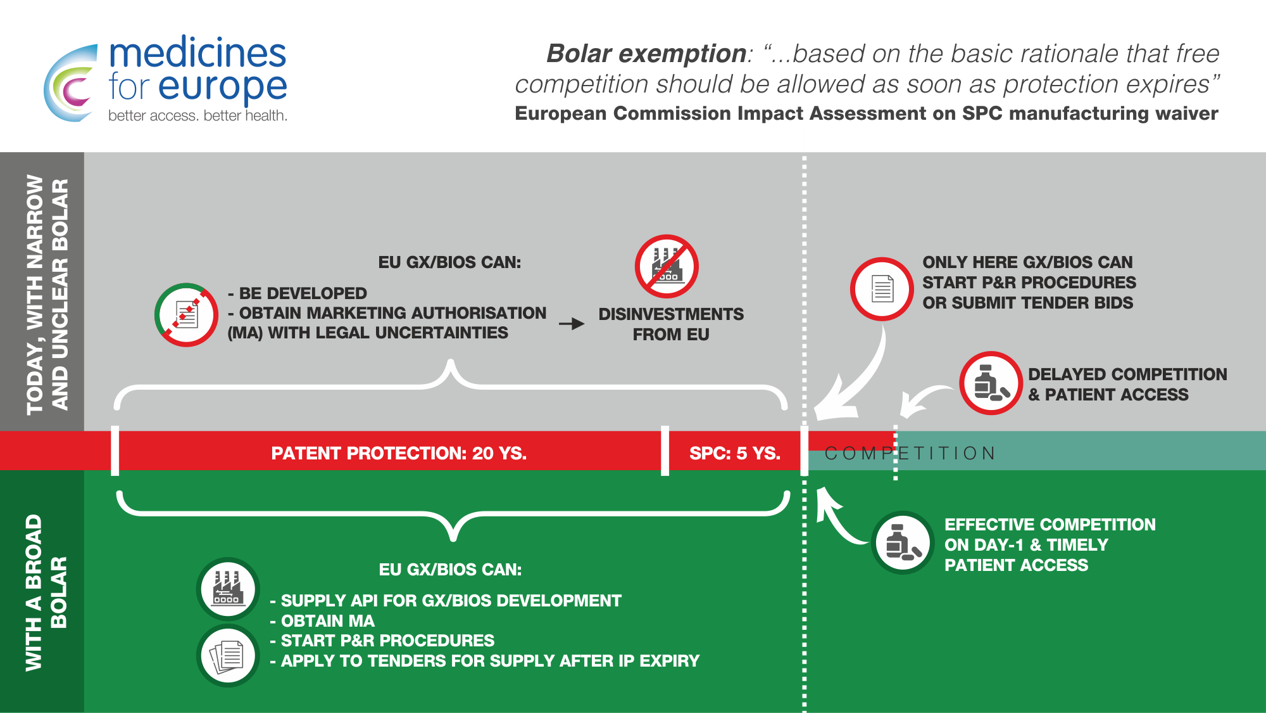
The competitiveness of the European industry con only be strengthened if the legislative framework related to the Bolar exemption is clarified and harmonised in the context of the EU pharmaceutical legislation review.
The Bolar exemption allows companies, during the patent/Supplementary Protection Certificate (SPC) protection of the reference product, to conduct studies, trials and the subsequent practical requirements necessary to obtain regulatory approvals for generic and biosimilar medicines, without this being considered patent/SPC infringement.
The primary objective of the Bolar is to “ensure that a generic could enter the market as soon as possible after the expiry of patent/SPC protection […] based on the basic rationale that free competition should be allowed as soon as protection expires”
(EC Impact Assessment on the SPC manufacturing waiver, p. 15)
However:
(i) Member States have provided different interpretations, leading to legal uncertainty for developers of generics, biosimilars and active pharmaceutical ingredients (API), driving investments on API development outside of Europe;
(ii) its restrictive interpretation blocks administrative procedures creating patent linkage (defined “unlawful” by the European Commission) and delaying generic/biosimilar market entry with huge economic consequences for healthcare budgets and patient access to medicines.
Therefore, the Commission, in pursuit of the primary purpose of the Bolar to ensure that generic & biosimilar medicines can enter the market on day-1 after IP expiries, should build on all studies and assessments already conducted and propose a revised & harmonised Bolar. It should also formally ban patent linkage in EU legislation.
The SPC manufacturing waiver, introduced in 2019 in EU legislation, is essential for the EU industry competitiveness. Its introduction is expected to create 25.000 direct jobs, €3.1 billion saving in EU pharmaceutical spending and net sales of the EU based pharmaceutical industry of €9.5 billion.
A smooth implementation of the SPC manufacturing waiver is key to stimulate more investments in Europe and avoid further technology transfer of pharmaceutical production outside Europe and boost R&D and manufacturing investment in Europe. Medicines for Europe, with its Member Companies, is monitoring potential obstacles in the implementation of the waiver and collects input from industry to inform the European Commission 5-year review expected in 2024. The review aims to assess whether all the conditionalities and limitations introduced in the SPC manufacturing waiver legislation actually allow the measure to produce the benefits it has been introduced for.
As announced in the European Commission Communication on a Pharmaceutical Strategy for the EU, a structured dialogue has been set up with the players in the pharmaceuticals manufacturing value chain, public authorities, patient and health non-governmental organisations and the research community. In its first phase, the structured dialogue will aim to gain a better understanding of the functioning of global supply chains and identify the precise causes and drivers of different potential vulnerabilities, including potential dependencies threatening the supply of critical medicines, active pharmaceutical ingredients and raw materials based on data collection and analysis.
In a second phase, the structured dialogue will serve to put forward a set of Key points and Extracts from the first meeting of the European structured dialogue on February 26th, 2021 which included measures to address the identified vulnerabilities and formulate policy options to be considered by the Commission and other authorities in the EU to ensure the security of supply and the availability of critical medicines, active pharmaceutical ingredients and raw materials. While it is important to assess whether manufacturing capacity for certain critical medicines may be required in the EU from the perspective of public health and crisis preparedness, any potential measures would have to be in full compliance with EU competition and World Trade Organization (WTO) rules.
Key points and Extracts from the first meeting of the European structured dialogue on February 26th, 2021:
“Participants agreed that the road towards robustness must not focus only on the current situation but also on looking ahead in order to prepare for the future. The ability to maintain and optimise open and well-functioning supply chains will require long-term policy measures to accelerate innovative and green production capacities. Participants also highlighted that the challenges to the manufacturing capability and capacity from the perspective of supply security depend on the form of products (solid, sterile/aseptic or complex molecules).
Speakers recommended considering a number of key topics within the work streams proposed. Namely:
Speakers also called for sustainable market conditions to improve resilience, investment in green production and reduction in environmental footprint.” European Commission official website.
Everyone agreed on the need for the Initiatives and on the need to “…better understand the vulnerabilities and dependencies that create strategic risks for critical supply in the EU on the ground” to bring positive change » (European Commission Vice-President Margaritis Schinas).
Important market reforms have been pointed out, such as “Making security of supply a criterion as important as price in public procurement.” (Ms. Nathalie Colin-Oesterlé, Member of the European Parliament).
The New European Pharmaceutical Strategy “…represents a great opportunity to put the patient at the centre of our policies and to attract and consolidate a competitive and diversified pharmaceutical industry in Europe with: a less bureaucratic and more efficient regulatory framework, without sacrificing security; market policies that facilitate the implementation of new scientific, technological and digital developments.” And with: “a new incentive system for: multiplying production capacities in Europe; creating a competitive ecosystem of manufacture supplying companies; maintaining the attractiveness of marketing essential medicines; covering unmet therapeutic needs.” (Ms Dolors Montserrat, Member of the European Parliament)
European Commission Structured Dialogue Initiative website Structured Dialogue initiative – Launch event | Public Health (europa.eu)
Keynotes from the European Commission first meeting of the European structured dialogue:
Opening speech by Vice-President Margaritis Schinas
Keynote speech by Commissioner Stella Kyriakides
Intervention by Portuguese Minister for Health, Marta Temido
Intervention by MEP Nathalie Colin-Oesterlé
Intervention by MEP Dolors Monserrat
Medicines for Europe statement on Industrial Policy addressed to the EUCO leaders